Publication 60
- Ir-Catalyzed Selective Hydroboration of 2-Substituted 1,3-Dienes: A General Method to Access Homoallylic Boronates
Daniele Fiorito, Clément Mazet*
ACS Catal. 2018, 8, 9382-9387
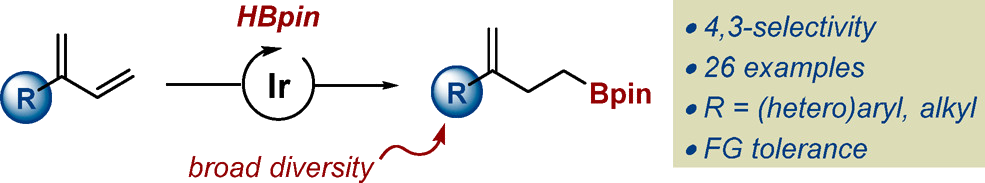
An operationally simple protocol for the 4,3-selective hydroboration of 2-substituted 1,3-dienes using an iridium catalyst is described. Independently of the nature (alkyl, aryl, heteroaryl) and the size of the substituent in 2-position, it provides access to a variety of homoallylic boronates featuring a 1,1-disubstituted olefin in high yield, chemo- and regioselectivity. An array of potentially sensitive functional groups is well-tolerated and the method can be extend to 1,2-disubstituted 1,3-dienes. Derivatization of the homoallylic boronates is also demonstrated using contemporary catalytic and enantioselective processes.
archive ouverte unige:107880
DOI de la version éditeur : 10.1021/acscatal.8b02334
DOI de l'AAM : 10.26037/yareta:c4y44m4cjzhgbnhlx2kycfyetu
DOI du Dataset : 10.26037/yareta:dawn4odedfa4zjtwgmeh2d2lbq
