Publication 75
- Nickel-Catalyzed Kumada Vinylation of Enol Phosphates: A Comparative Mechanistic Study
Philippe-Alexandre Poisson, Gaël Tran, Céline Besnard, Clément Mazet*
ACS Catal. 2021, 11, 15041-15050
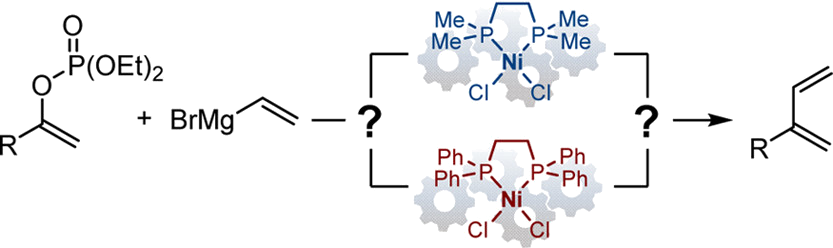
Based on supporting stoichiometric organometallic syntheses, structural analyses, reaction monitoring, radical-clock experiments, and kinetic investigations, a comparative mechanistic study between two related systems that are competent in the Ni-catalyzed Kumada cross-coupling reaction between enol phosphates and vinyl magnesium bromide is reported. We demonstrate that the two bisphosphine–nickel complexes operate via Ni(0)/Ni(II) catalytic manifolds for this type of C(sp2)–O electrophiles. The first complex of generic structure [(dppe)NiBr2] is reduced in situ upstream from the productive catalytic cycle, which itself follows a classical mechanistic scenario composed of an oxidative addition/transmetalation/reductive elimination sequence. A rapid phosphate/bromide ion exchange prior to transmetalation constitutes a key feature of this system. The related [(dmpe)NiBr2] complex follows a much less conventional pathway. Kinetic analyses distinguish themselves by an unusual apparent second order in the catalyst, which is attributed to an interplay between vinyl nickel species formed during precatalyst activation and styrenyl nickel intermediates generated downstream in the sequence of elementary steps. This hypothesis is corroborated by independent supporting organometallic investigations.
archive ouverte unige:157354
DOI de la version éditeur : 10.1021/acscatal.1c04800
DOI de l'AAM : 10.26037/yareta:yni4rgcfrfgebbrw6323r5qcpm
DOI du Dataset : 10.26037/yareta:npyrv7klrbfijgvvigw7lzpeh4
