Publication 66
- A Catalytic Dual Isomerization/Allylboration Sequence for the Stereoselective Construction of Congested Secondary Homoallylic Alcohols
Yangbin Liu, Clément Mazet*
J. Org. Chem. 2020, 85, 5638-5650
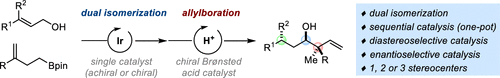
A catalytic sequence for the diastereo- and enantioselective preparation of homoallylic alcohols with an adjacent quaternary (stereo)center is reported. The one-pot process relies on the use of a single (achiral or chiral) iridium complex to catalyze the concomitant isomerization of primary allylic alcohols and homoallylboronates into (chiral) aldehydes and allylboronates respectively. In the same flask, a chiral Brønsted acid is added next to engage the isomerization products into a stereocontrolled allylboration reaction. Structural variations have been performed on both the allylic alcohols and the homoallylboronates. This mild process affords an array of stereochemically congested and complex chiral secondary homoallylic alcohols in high yield, excellent diastereoselectivity and usually high enantioselectivity.
archive ouverte unige:134731
DOI de la version éditeur : 10.1021/acs.joc.0c00565
DOI de l'AAM : 10.26037/yareta:wx2ql65ytba6zliewk35263loy
DOI du Dataset : 10.26037/yareta:rb6tfhh6yjcidgxuan5auvmo4m
